Antigen Rapid Test Kit (ARTRON)
COVID-19 Antigen Rapid Test Kit

Product characteristics
Artron COVID-19 Antigen Test is a rapid and convenient immuno-chromatographic assay for the qualitative detection of COVID-19 antigen (viral nucleoprotein) from Nasopharyngeal or nasal
swab from patient with signs and symptoms of respiratory infection. The test kit is designed to aid in the rapid diagnosis of COVID-19 Virus infection. This assay is not intended to be used for screening patients or as an aid for diagnosis of patients with suspected COVID-19 Ag infection, this assay is not intended for home testing (or self-testing). The results should be confirmed by Real-Time Reverse Transcriptase (RT)-PCR Diagnostic kit; they do not preclude COVID-19 Virus infection and should not be used as the sole basis for treatment or other management decisions.
- Nasopharyngeal or nasal
swab sample - Simple: Easy to perform, Solid phase (Collodial Gold) Immunochromatographic assay.
- Qualitative membrane-based immunoassay (Colloidal Gold). A colored line will appear in the respective line region. Test is positive if the control line and the antigene line shows up.
The test is intended for medical professional and laboratory use.
Indication for Testing
- To detect infection after contact with COV-2 infected persons
- At or before onset of symptoms in addition to PCR test.
- As a precaution before meeting third party
- If no PCR test is accessible.
- As a precaution in case of uncertainty if infection took place.
- As a follow up after being PCR positive tested, to monitor the status if potentially still infective.
Specifications
- Sensitivity: 96,7%
- Specificity: 100%
Order Number:
Nasopharyngeal Swab
S-CoV-2- 422PNP25
Nasal
S-CoV-2- 422PNS25
(Kit with 25 tests)
Price: From 3,50€ per test
| No | Content | Quantity |
|---|---|---|
| 1 | Instructions for use | 1 copy |
| 2 | Test cassettes with desiccant in individual pouch | 25 devices for 25 pcs/pack. |
| 3 | Plastic ampoules sealed with 420μL sample buffer | 25 ampoules for 25 pcs/pack. |
| 4 | Extraction tube caps | 25 pcs/pack |
| 5 | Sterilized nasopharyngeal or sterilized nasal swaps | 25 pcs/pack |
| 6 | 1 tube rack for 25 pcs/pack |
| Product Name | Sample Type | Storage Temperature | Packaging Size |
|---|---|---|---|
| COVID-19 Antigen Rapid Test Kit | Nasopharyngeal or nasal swab sample | Store at 2°C -8°C | 25 Tests / Kit |
Specimen Collection

Specimen Collection

1
Insert a sterile swab into the nostril of the patient, reaching the surface of the posterior nasopharynx.
2
Swab over the surface of the posterior nasopharynx.

3
Withdraw the sterileswab from the nasal cavity.
Test Procedure

A
Open the plastic ampoule by twisting off the top and squeeze the buffer into the extraction tube till to the fill-line of the extraction tube.

B
Insert the swab in the extraction tube. Swirl the swab tip vigorously in the buffer fluid at least 10 times.

C
Remove the swab by rotating against the extraction tube while squeezing the sides of the tube to release the liquid from the swab. Properly discard the swab.

D
Close the extraction tube with the provided extraction tube cap and push firmly onto the tube.

E
Add
Get the test cassette from the sealed pouch by tearing at the notch and place the cassette on a flat, dry surface.

F
Vigorously Hold the extraction tube vertically above the sample well, slowly add 4 drops of the specimen without air bubbles into the sample well. DO NOT touch the card with the dropper tip while dispensing.

G
Read and interpret the test result within 15-30 minutes. The test result should not be read and interpreted after 30 minutes.

D
All used test components should be disposed of in Biohazard Container.
Result Interpretation
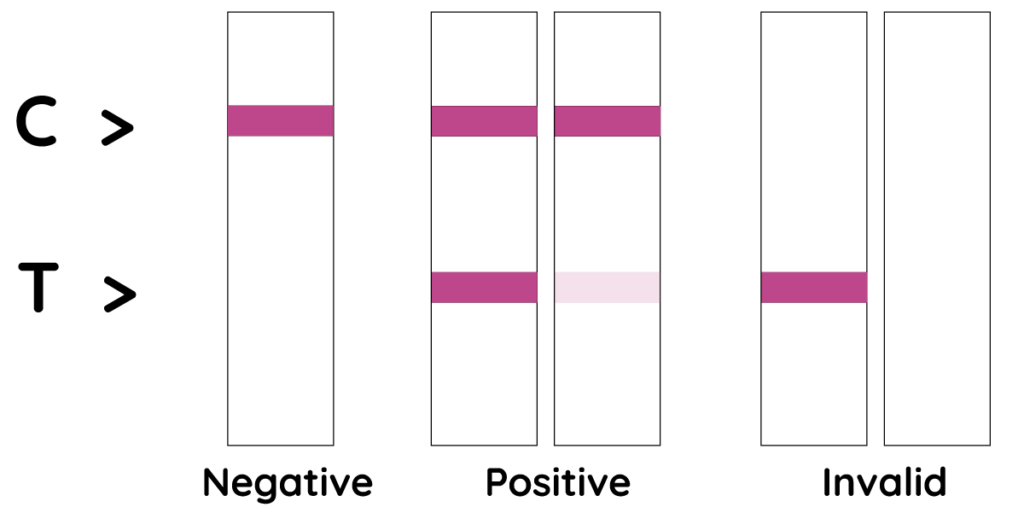
Negative:
A pink colored band appears only at the control region (C), indicating a negative result for COVID-19 Infections.
Positive:
A clear pink control band (C) and a detectable test band (T) appears, indicating a positive result for COVID-19 infections.
Invalid:
No visible band appears at the control region. Repeat with a new test kit. If the test still fails, please contact the distributor with the lot number.
